Our research is quite diverse, and it exemplifies the notion that 'structure is an embodiment of reactivity and other attributes such as molecular organiza-tion'. The importance of 'structure' bears out in every domain of our research activity, namely, i) organic photochemistry, ii) supramolecular chemistry and iii) mechanistic organic chemistry. In the area of photochemistry, based on sterics and electronic factors in rationally designed molecules, we endeavor to control the reactivity/ phenomenon. The diastereo - diffe-rentiatingphotoreactivity that we have unraveled for ketones with two contiguous stereogenic centers has led to unprecedented insights concerning the well-known Norrish Type II reactions and the behavior of reactive 1,4-biradicals in general. In our recent studies, we have shown how helicity and p-conjugation may modify the photophysical (fluorescence) property and photochromic phenomenon. In the realm of supramolecular chemistry, our research focus, in addition to the efforts on understanding intermolecular interactions, is centered on controlling molecular ordering by a rational design at the molecular level. In particular, we are intensely pursuing the development of organic functional mimics of inorganic zeolites, i.e., MOFs, for a variety of applications. By exploiting the concepts of supramolecular chemistry in molecular design, we have been focused on developing amorphous organic materials for application in organic light emitting diodes (OLEDs). Insofar as mechanistic organic chemistry/organic synthesis is concerned, we have been interested in understanding the reactivity of IBX, o-iodoxybenzoic acid, which has emerged as a remarkable oxidation reagent in the last 15 years. We continue to develop modified IBXs with improved solubility and controlled reactivity. Development of catalytic and chiral IBXs constitutes our present focus.
-
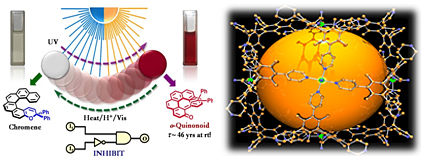
Helicity as a Steric Force: Stabilization and Helicity-Dependent Reversion of Colored o-Quinonoid Intermediates of Helical Chromenes, J. Am. Chem. Soc., 135, 6872 (2013).
-
Twist Does a Twist to the Reactivity: Stoichiometric and Catalytic Oxidations with Twisted Tetramethyl-IBX, J. Org. Chem., 76, 9593 (2011).
-
Enantioselective Organocatalytic Biginelli Reaction: Dependence of the Catalyst on Sterics, Hydrogen Bonding, and Reinforced Chirality, J. Org. Chem., 76, 396 (2011).
-
Intramolecular O-H•••O Hydrogen Bond-Mediated Reversal in the Partitioning of Conformationally-Restricted Triplet 1,4-Biradicals and Amplification of Diastereo differentiation in their Lifetimes, J. Am. Chem. Soc., 130, 13608 (2008).
-
A De Novo Design for Functional Amorphous Materials: Synthesis, Thermal and Light Emitting Properties of Twisted Anthracene-Functionalized Bimesitylenes, J. Am. Chem. Soc., 130, 17320 (2008).
-
Corundum, Diamond, and PtS Metal–Organic Frameworks with a Difference: Self-Assembly of a Unique Pair of 3-Connecting D2d-Symmetric 3,3',5,5'-Tetrakis(4-pyridyl)bimesityl, Angew. Chem. Int. Ed., 44, 2415 (2005).
-
Professor, IIT Kanpur, 2008-.
-
Associate Professor, IIT Kanpur, 2003-2007
-
Asst. Professor, IIT Kanpur, 1998-2003
-
Asst. Professor, IIT Kharagpur, 1998
-
Univ. of Victoria, Canada, 1996-1998
-
Univ. of Wuerzburg, Germany, 1995-1996
-
Postdoctoral Fellow, Univ. of Houston, USA, 1994-1995
-
J. C. Bose fellowship from DST (2015).
-
Dr. Jag Mohan Garg Chair Professor, 2015-2018
-
Royal Society of Chemistry, 2014
-
Lalit M. Kapoor Chair Professor, 2011-2014
-
Fellow, Indian Academy of Sciences, 2010
-
Ramanna Research Fellowship, 2007-2010
-
Shanti Swarup Bhatnagar Prize, 2008
-
CRSI Young Chemist of the year, 2004
Office
CL 204A ,
Department of Chemistry
IIT Kanpur,
Kanpur 208016
Office Phone: 0512-259-7438 (O)
Email: moorthy[AT]iitk.ac.in
|
|
|












